WHAT IS ISO 13485 ?
ISO 13485 is an International Quality Management System standard for any company involved in the design, production, installation, servicing and manufacturing of medical devices. ISO 13485 was first published in 1996 and has since been revised in 2003 and 2016. The current version, ISO 13485:2016, came into effect in March 2016. The aim of these requirements is to ensure that medical devices and services consistently meet customer expectations and relevant regulatory requirements. Its primary objective is to facilitate harmonised medical device regulatory requirements. The standard contains specific requirements for manufacture, installation and servicing of medical devices:
- Implementation of effective Quality Management System.
- Risk Management Approach to product development and product realization.
- Validation of Processes.
- Compliance with Statutory and regulatory requirements
- Effective product traceability and recall systems.

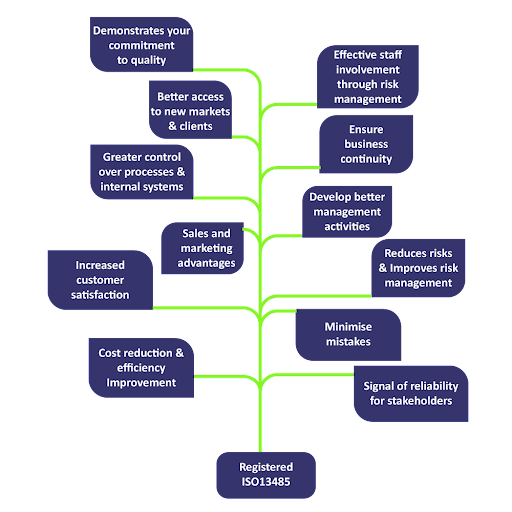
WHY TO CHOOSE ISO 13485?
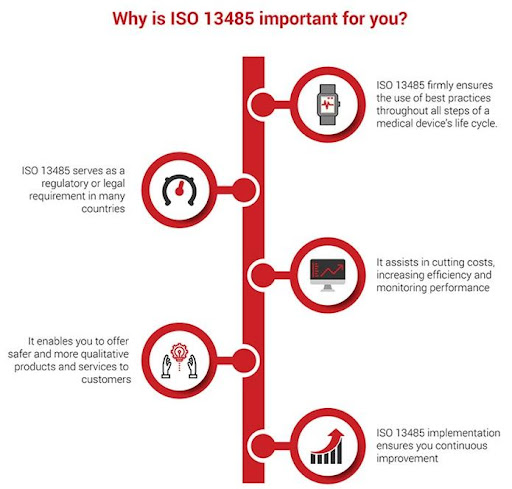
HOW MUCH DOES ISO 13485 COST?
The cost of certification depends on several factors. These include your business sector, type of organization, your scope of work, and your number of employees. Moreover, costs vary as per industry specific accreditation as well (such as from EGAC, EIAC, IAS, DAKKS, UKAS, JAS-ANZ or NABCB).
However, QA INTERNATIONAL SERVICES is committed to provide a cost-effective route to ISO 13485 certification. That’s why we focus on making ISO certification simple and straightforward. We save you money because we spend less time creating complex document trails and more time building a system that works for your business.
AN ISO 13485 AUDIT?
An ISO 13485 audit helps determine the actual status and health of your current QMS and processes. The purpose of quality audits is to verity that manufacturing, development, and related control facilities meet current good manufacturing processes (GMP), as well as conform to the commitments of ISO 13485. Medical device manufacturers are required to perform regular audits of their ISO 13485 compliant Quality Management System (QMS). Internal audits support the safety and effectiveness objectives of the products they sell and demonstrate that an adequate, effective quality system is established and maintained. Your quality staff can perform these audits, but a professional, third-party auditor is more objective when assessing the status of your QMS and processes.
An ISO 13485 audit includes:
- An off-site review of your current quality and regulatory documentation before your on-site ISO 13485 audit by a Notified Body or Registrar.
- A systematic and independent process audit to determine conformity or nonconformity of your QMS to ISO 13485:2016 requirements.
- A review of your internal and external documentation to verify requirements have been addressed.
- An actual verification of ISO 13485 audit requirements through a review of the objective evidence.
- A physical verification of compliance via interviews and fact-based observations to confirm the quality system requirements are met.
- An evaluation of current special controls and validated processes.
BENEFITS OF ISO 13485 FOR YOUR ORGANISATION
The ISO 13485:2016 (Medical Devices Quality Management System) specifies what your organisation needs to do:
- To provide medical devices and services that consistently meet customers’ needs
- To enhance customer satisfaction through a processes of continual improvement
- To ensure it conforms with all applicable statutory and regulatory requirements
Becoming certified to ISO 13485:2016 (MDQMS) can provide many benefits, both for your organisation and your customers.
- International recognition, especially in major global markets
- Legal compliance based on local regulations
- Bringing quality and continuous improvement into the heart of your medical device organization.
- Improved product safety and quality
- Improved patient/customer satisfaction – by consistently providing safe medical devices that meet customer requirements.
- Enhanced reputation and credibility – by being ISO 13485:2016 accredited, you can demonstrate to customers, suppliers and other stakeholders that your organization is serious about quality.
- Greater efficiency – ISO 13485:2016 can help streamline your processes, making your organization more efficient overall.
- Reduced costs – ISO 13485:2016 can lead to reduced waste, rework and other inefficiencies, thereby reducing your organization’s overall costs.
- Improved risk management – by having a robust quality management system in place, you can more effectively identify and manage risks associated with your medical devices
- A stronger foundation for growth – ISO 13485:2016 can provide a solid foundation for your organization to grow and expand its operations into new markets.